Answers for yesterday's homework. How did you do? Are you a super genius? 😊
You didn't need to include the OBN, but I did 'cause I'm awesome like that.
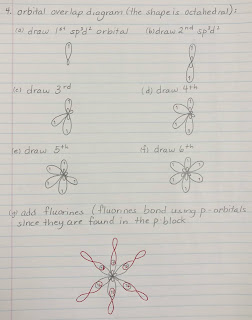
Notice that the Cl and the F use half-filled p orbitals to overlap with the central atom's hybrid orbitals (unlike H which uses an s orbital).
See the end of this post for a more detailed explanation of phosphorus pentachloride.
To briefly summarize what we have seen thus far:
sp = linear
sp2 = trigonal planar
sp3 = tetrahedral, trigonal pyramidal, bent
sp3d = trigonal bipyramidal
sp3d2 = octahedral
These VSEPR shapes are always linked to these particular orbitals. ALWAYS.
Multiple Bonds
To date we have been forming single covalent bonds through the overlap of orbitals. This is called a sigma bond (σ-bond).
To describe multiple bonds, we consider a bond that results from the overlap of p orbitals perpendicular to the σ-bond axis. This produces a pi bond (𝜋-bond).
A double bond contains a sigma bond and a pi bond. A triple bond contains a sigma and two pi bonds.
Double bonds are difficult to rotate and require a large energy input. Triple bonds are almost impossible to rotate. Although, we don’t need this rotation info right now, it will come in handy in a future unit.
Let's start by looking at ethene, which contains a C=C double bond.
We will focus on one of the two Cs in ethene, since they are undergoing the same process.
Notice that the GSEC and the promotion are the same as they were in methane (from the last lesson). We still need four equivalent half-filled orbitals because we are still creating four bonds (2 singles and a double).
Notice that each C has a trigonal planar arrangement of bonds around it. Therefore, for the hybridization, we wish to create three sp2 orbitals (sp2 = trigonal planar). So, the s, px and py orbitals are mixed to make the three sp2 hybrid orbitals. This leaves a pz orbital unaffected (don't worry - he has a job to play later).
To create the orbital overlap diagram, draw the three sp2 orbitals in a trigonal planar shape for the first C and the mirror image of this for the second C. One of the sp2 orbitals on each C should overlap in the middle, to create the C-C σ-bond. Although, it is not shown in my diagram, you should overlap the half-filled 1s orbitals of the four Hs with the remaining sp2 orbitals.
Now, what about the left over pz orbital on each C? These orbitals will orient themselves perpendicular to what we have already drawn. the top lobes and the bottom lobes of the two half-filled pz orbitals will overlap to create a C=C 𝜋-bond (this is represent by the oval shape above (overlapped upper lobes and below (overlapped lower lobes).
Here's a video that I made to take you through this.
TryIt! Using the logic above, show how the triple bonding in ethyne works. Hint, notice the linear shape around the C. The answer is directly below and I made a video too.
Homework:
Although the HW chart does not have a column for the orbital overlap diagrams, you will want to practice at least a few. I have included this in the answer keys so you can check yourself.