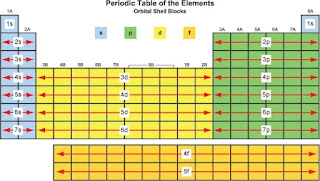
Explaining the Periodic Table
Notice that the maximum number of electrons in the s, p, d and f orbitals correspond exactly to the number of columns in the s, p, d and f blocks on the periodic table.
Now, unlike with the Bohr model, we have a better idea of how the electrons in an atom, beyond the first twenty, are arranged.
Explaining Ion Charges
Up til this point, we have not been able to explain transition metal ions or multivalent elements. Now, many ions can be explained.
For instance look at zinc, Zn: [Ar]4s23d10, which shows 12 outer electrons.
If the two high energy 4s electrons are removed, the Zn ion will have a full outer shell that is stable, Zn: [Ar]3d10.
In this case it makes more sense to lose the two higher energy electrons, than to lose two 3d electrons and leave that orbital partially filled.
For a multivalent element like Pb: [Xe]6s24f145d106p2, the element could lose the two 6p electrons to form the 2+ ion or it could lose the 6p and the 6s electrons to form the 4+ ion.
Losing the pairs of electrons makes more sense than removing 4f or 5d electrons which would leave these orbitals partially filled.
Explaining Magnetism
Look
at the electron configurations of three ferromagnetic (strongly magnetic)
materials (I have shown the orbital box notation for the 3d orbitals only):
It is tempting to think that ferromagnetic materials are only a result of unpaired electrons.
But, Ru, Rh and Pd, directly below Fe, Co and Ni on the PT have the same d-orbital configurations, but are only paramagnetic (weakly magnetic).
So, unpaired electrons are part of the reason, but we must look elsewhere for a full explanation.
It has been found that ferromagnetism depends on the ability of a group of atoms to form domains which align themselves in the same direction.
Analogy time: Imagine we, as a class, decide to go to the movies. When we enter the empty theatre, groups of students will likely sit together (domains), focusing on the same thing. One group might face front to watch the previews, another group might face the side to watch the cute employee walking into the theatre and another group might be turned around staring at the group at the back, which is throwing popcorn. This is like (a) in the figure above - electrons are aligned within the pockets or domains, but the domains are not aligned with each other. These unaligned domains weaken each other's magnetic fields. This results in no/weak magnetism.
If we go back to the theatre as the curtain rises and the movie starts, all students, regardless of the group in which they are sitting, will swivel to face the front. This is like (b) in the figure above. Now, all the domains are aligned. This orientation allows for the magnetic fields to augment each other. This results in strong magnetism.
Anomalous Electron Configurations
Predictions on the electron configurations of the first row transition metals, above the experimental evidence.
Notice, that there are two exceptions, Cr and Cu.
From previous chemistry learning, we know that filled and empty subshells are quite stable (😃). Now, we will realize that half-filled subshells have a certain stability (🙂) as well. Any other type of filling is not as stable (😔).
In the case of Cr, an s electron is promoted to the d orbital to create two half filled subshells.
In the case of Cu, an s electron is promoted to the d orbital to create a half filled s subshell and a filled d subshell.
Based on experimental evidence, this is the lowest possible energy state for these two atoms.
Homework # 11-17