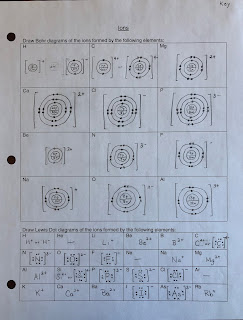
Ions
An ion is a charged particle formed when an atom loses or gains electrons.
Atoms lose or gain electrons to attain a full outer shell like the Noble Gases (this is known as the Octet Rule).
Metals
When atoms lose electrons, there are now more protons than electrons, so the ion has
a positive charge. Typically, metals lose electrons to attain a full outer shell.
Potassium
- starts with 1 electron in its outer shell
- it could gain 7 electrons or lose 1 electron to attain a full outer shell
- it makes more sense to lose 1 electron
- this gives potassium a positive charge (+)
Magnesium
- starts with 2 electrons in its outer shell
- it could gain 6 electrons or lose 2 electrons to attain a full outer shell
- it makes more sense to lose 2 electrons
- this gives magneisum a positive charge (2+)
Non-metals
When atoms gain electrons, there are now more electrons than protons, so the ion has a negative charge. Typically, non-metals gain electrons to attain a full outer shell.
Chlorine
- starts with 7 electrons in its outer shell
- it could gain 1 electron or lose 7 electrons to attain a full outer shell
- it makes more sense to gain 1 electron
- this gives chlorine a negative charge (-)
Phosphorus
- starts with 5 electrons in its outer shell
- it could gain 3 electrons or lose 5 electrons to attain a full outer shell
- it makes more sense to gain 3 electrons
- this gives phosphorus a negative charge (3-)
Isoelectronic
Two particles are said to be isoelectronic if they have the same number of electrons. Typically, we will find out with which Noble Gas the ion is isoelectronic.
Looking at the ions above:
- K+
is isoelectronic with Ar (since both K+ & Ar have 18 total electrons)
- Mg2+ is isoelectronic with Ne (since both Mg2+ & Ne have 10 total electrons)
- Cl- is isoelectronic with Ar (since both Cl- & Ar have 18 total electrons)
- P3- is isoelectronic with Ar (since both P3- & Ar have 18 total electrons)
Question of the Day #14:
For sulfur and lithium:
(a) Draw the Lewis diagram for each atom.
(b) Draw the Lewis diagram for each ion.
(c) Indicate with which Nobel Gas each ion is isoelectronic.
Homework:
Answers: