The Bohr-Rutherford Atom
The Bohr-Rutherford atom consists of a central nucleus, surrounded by progressively larger concentric circles. This model can be likened to the solar system.
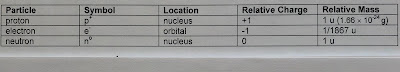
Subatomic Particles
There are three subatomic particles, that combine in various amounts, to create an element.
Standard Atomic Notation (AZX)
Standard atomic notation is one method used to communicate the components of a given element.
Bohr – Rutherford Diagrams
 Bohr- Rutherford diagrams (or Bohr diagrams, for short) are used to illustrate the components of a given element.
Bohr- Rutherford diagrams (or Bohr diagrams, for short) are used to illustrate the components of a given element.
Recall:
- typically, we only draw these diagrams for the first twenty elements on the Periodic Table
- the maximum number of electrons in each shell is 2, 8, 8, 8 - moving outward from the nucleus
- in the second shell and beyond, the electrons are never doubled up until each of the four spaces already hold one electron
Lewis Diagrams
Lewis diagrams consist of the element symbol with the appropriate number of dots to represent valence electrons.
Recall:- these diagrams can be drawn for any element on the Periodic Table
- we only show the outermost (valence) electrons
- electrons are never doubled up until each of the four spaces already hold one electron
- as we move across the tall columns on the Periodic Table (from left to right), the number of valence electrons follows this pattern: 1, 2, 3, 4, 5, 6, 7, 8
Mendeleev’s Periodic Law
Mendeleev's periodic law states, "If the elements are arranged according to their atomic mass, a pattern can be seen in which similar properties occur regularly."
Mendeleev's journey to create the periodic table is fascinating.
Modern Periodic Table
Modern periodic law states, "If the elements are arranged according to their atomic number, a pattern can be seen in which similar properties occur regularly."
Periodic Table
Label:
➤ staircase
➤ periods (rows); period numbers (1 to 7)
➤ families/groups (columns); group numbers (1 to 18)
- alkali metals
- alkaline earth metals
- transition metals
- aluminum group
- carbon group
- pnictogens
- chalcogens
- halogens
- noble gases
- lanthanides
- actinides
Metals, Non-metals & Metalloids
- metals – malleable, ductile, conductive, shiny (ex. Na, Pb, Hg)
- non-metals – brittle, non-conductive, dull (ex. N, S, Ar)
- metalloids – have properties of both metals and non-metals (ex. Te, Si, Te)
Homework:
Draw the Bohr diagrams for the first twenty elements.
Draw the Lewis diagrams for the first twenty elements.
Answer Key: