You will be carrying out an individual, week-long titration lab. You will be assigned a station to work at and you will set up all your equipment and perform the experiment in this space.
Equipment:
Collect and clean (as appropriate) the following equipment:
- Winchester bottle - found under the student sinks on the right side of the room.
- burette - front of room behind cart
- burette clamp - cart
- retort stand - cart
- funnel - cart
- Erlenmeyer flask (any size) x 3 - cart
- beaker (small) - cart
- phenolphthalein - cart
- 10 mL pipette - cart
- pipetter - cart
- white paper - BYOWP
Sections:
(I) Solution Preparation
- prepare 500.0 mL of a 0.10 M NaOH solution
- use the procedure you learned when we talked about creating a solution from a solid solute
(II) NaOH Standardization
- since NaOH(s) is hygroscopic, it is impossible to make a concentration of exactly 0.1 M
- in this section you will determine the actual concentration of the NaOH solution the you made in part (I)
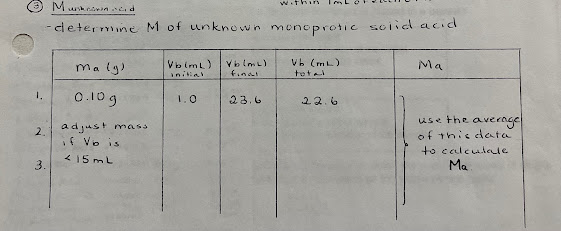
(III) Molar Mass of Unknown Monoprotic Solid Acid
- in this section, you will determine the molar mass of monoprotic, solid unknown acid
Assessment:
- Report - include NaOH mass calculation (part (I)), NaOH standardization chart & calculation (part (II)) and molar mass chart & calculation (part (III)). /10 marks
- Accuracy - NO CODE = NO MARK /20 marks
- Technique /10 marks
- Clean-Up /5 marks