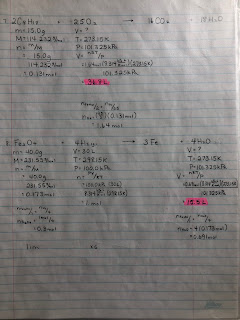- Nomenclature! Nomenclature is here to stay, baby. Learn to love it.
- FORMAT! FORMAT! FORMAT! The format I taught you is still required. Do not skip steps - every single bit of format is required for full marks. The final answer is worth ONE mark.
- know the topics in "Gases" (the very first lesson)
- be able to convert between the different units for pressure
- Gas Laws (Boyle's, Charles', Gay-Lussac's, Combined, Dalton's, Ideal, Avogadro's) ↝ state them, understand them, use them for calculations
- convert temperature in Celsius to Kelvin and vice versa
- STP, SATP ↝ state them, use them for calculations
- Law of Combining Volumes, Law of Multiple Proportions ↝ state them, understand them
- know how to do the calculations involving density, molar mass and empirical/molecular formulae
- real vs ideal gases ↝ similarities/differences
- stoichiometry - both flavours
- know the topics in "Atmospheric Reactions & Pollution"
Review: HINT: the chemical formula for acetylene is C2H2(g).
Answers: