Alkanes are fairly unreactive and therefore undergo very few reactions.
- Combustion Reaction
Alkanes, as well as all other organic compounds, can undergo combustion.
2C8H18(l)
+ 25O2(g) → 16CO2(g) + 18H2O(g)
- Substitution Reaction - Halogenation (with F2, Cl2 or Br2)
The hydrogen atom(s) in an alkane may be
substituted by a halogen atom, using F2, Cl2, Br2. The reactions using F2 are
vigourous, while the reactions using Cl2 and Br2 require
heat (Δ) or ultraviolet (UV) light to first dissociate the halogen molecule before
the reaction will proceed. The product
of this reaction is called an alkyl halide, which is given the general formula R-X, where R is any hydrocarbon and X is any halogen atom.
 |
| The conditions (which are always found above the arrow) will dissociate the bromine molecule into two separate Br atoms. One of the Br atoms will attack a H in the hydrocarbon. The H leaves, allowing the Br atom to substitute into that position. The remaining Br and the ousted H join together. Thus, the products of a substitution reaction are always an alkyl halide (RX) and a hydrogen halide (HX). Further substitutions may occur, resulting in further Hs being replaced by further Xs (as seen in the second step). A video sure would be helpful. |
(II) Reactions of Alkenes & Alkynes
Molecules with double or triple bonds between carbon tend to be quite reactive. Thus, these reactions often occur readily at room temperature, although sometimes other reaction conditions must be employed.
- Addition Reaction – Halogenation (with Br2 or Cl2)
 |
| The bromine molecule will attack the alkene at the double bond, resulting in the double bond popping open (leaving a single bond between those two Cs). Subsequently, the bond between the two Br atoms breaks. The two C atoms (that were previously involved in the double bond) bond to the Br atoms. Did someone say video? |
- Addition Reaction – Hydrogenation (with H2)
 |
| Substitution reactions can also occur with alkynes. In this case, one of the hydrogen molecules will attack the alkyne at the triplebond, resulting
in the triple bond popping open (leaving a double bond between those two
Cs). Subsequently, the bond between the two Hr atoms breaks. The two C
atoms (that were previously involved in the triple bond) bond to the H
atoms. The process repeats itself, with the second hydrogen molecule attacking the remaining C=C double bond, ultimatley creating an alkane. The conditions for a hydrogenation always require heat and pressure, as well as a catalyst (although the catalyst varies depending on the hydrocarbon undergoing the addition). I wonder if there's a video? |
- Addition Reaction – Hydrohalogenation (with hydrogen halides)
 |
| A hydrohalogenation reaction follows the same pattern as the above addition reactions. I could really go for a video right about now. |
- Addition Reaction – Hydration (with H2O)
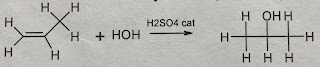 |
| A hydration reaction follows the same pattern as the above addition reactions. In this case, the conditions require the use of concentrated sulfuric acid as a catalyst. You will find as we proceed through this unit that typically conc sulfuric acid is the catalyst of choice when water is involved in the reaction. I bet that fab chem teacher of ours has a video for this. |
Markovnikov’s Rule
When molecules like H-H or Br-Br are added to a double bond, only one product is possible. However, when asymmetrical molecules like H-Br or H-OH are added to a double bond, two products are possible. Check it out.
When molecules like H-H or Br-Br are added to a double bond, only one product is possible. However, when asymmetrical molecules like H-Br or H-OH are added to a double bond, two products are possible. Check it out.
Markovnikov’s Rule states that ‘when a hydrogen halide or water is added to an alkene or alkyne, the hydrogen atom generally bonds to the carbon atom within the double bond that already has more hydrogen atoms.”
(III) Reactions of Aromatic Hydrocarbons
The reactivity of aromatic hydrocarbons is intermediate between that of alkanes and alkenes. This is because benzene does not truly have single or double bonds, but rather 1½ bonds.
Substitution reactions of benzene proceed in the same way as the substitution reactions of an alkane.
- Substitution – Halogenation (with Cl2 or Br2)
- Substitution – Nitration (with HNO3)
- Substitution – Alkylation (with an R-X (alkyl halide))
Homework #6-15


