Alcohols (R-OH)
If one of the hydrogens in a hydrocarbon is replaced with a hydroxyl group, -OH, an alcohol is formed. Some common alcohols are:
- ethanol, in wine and beer
- cholesterol
- retinol, a form of vitamin A used in face serums to reduce the appearance of wrinkles
- antifreeze, which is sweet tasting but toxic
- glycerin, which is non-toxic and is used in cosmetics and foods like chocolate
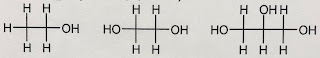 |
| ethanol antifreeze glycerin |
Hydrogen Bonding
Hydrogen bonding can occur between molecules that have H directly bonded to F, O or N.
Water can H-bond - the ẟ+ H in one molecule are attracted to the ẟ- O in an adjacent molecule.
This
intermolecular force is responsible for many of water’s properties:
- Water has a high boiling point - it takes a lot of energy to break the H-bonds between the water molecules ➣ H2O (which can H-bond) 100ºC vs H2S (which cannot H-bond) -61ºC.
- Water dissolves polar molecules – remember “like dissolves like.”
- Electrolytes (substances that break into ions – ionic compounds) also dissolve in water – each ion is surrounded by a sphere of hydration (see figure 8, p. 273).
- Since water is such a good solvent, ground water can be easily contaminated by many substances.
Physical Properties of the Alcohols
The physical properties of the alcohols are quite different from the parent alkane. The hydroxyl group, –OH, is polar (ΔEneg = 3.5 - 2.1 = 1.4) and is capable of hydrogen bonding.
Alcohols are attracted to one another and this causes a higher boiling point for the alcohol as compared to the analogous alkane.
Alcohols tend to be quite soluble in water as well, due to the attraction between the –OH in the alcohol and the water.
Alcohols also have high melting points and boiling points compared to the parent alkane.
Ethers (R-O-R′)
An ether was formerly used as an anesthetic (diethyl ether).Ethers can either be symmetrical (R-O-R) or asymmetrical (R-O-R′), depending on whether the alkyl groups attached to the O are the same or different.
Water is H-O-H, an alcohol is R-O-H and now an ether is R-O-R′. Both of the hydrogen atoms in water are replaced by alkyl groups.
Physical Properties of Ethers
The
C-O-C bonds are polar, so ethers will dissolve in water and have relatively high
melting and boiling points. However, ethers cannot H-bond like alcohols, so all these properties are less pronounced for ethers compared to alcohols.
Homework:
Section Questions, p. 208 # 1, 4, 5a, 6
Read "Explore an Issue" p. 202-203 then do p. 203 # 1-4.
Answers:


