Aldehydes (R-CO-H) & Ketones (R-CO-R′)
Molecules in both the aldehyde and ketone families
contain the carbonyl group (C=O). In aldehydes, the carbonyl group is always found at the end of the carbon chain. In ketones, the carbonyl group is found at any position other than at the end of the chain.
In nature, ketones are used by animals to send signals: ants use heptan-2-one to warn of danger and pheromones are used as sexual attractants by many animals.
One of the best known aldehydes is formaldehyde, used as an antiseptic and disinfectant. Acetaldehyde is used as a preservative and in the production of resins and dyes.
In nature, ketones are used by animals to send signals: ants use heptan-2-one to warn of danger and pheromones are used as sexual attractants by many animals.
One of the best known aldehydes is formaldehyde, used as an antiseptic and disinfectant. Acetaldehyde is used as a preservative and in the production of resins and dyes.
Naming
Aldehydes
Aldehydes are named by dropping the ending of the name of the longest chain and adding ‘-al. The numbering always places "1" on the carbon in the carbonyl group and the rest of the chain is numbered from there. Since the carbon in the carbonyl group is always designated as "1" there is no need to indicate the location of the carbonyl group in the name.
Aldehydes are named by dropping the ending of the name of the longest chain and adding ‘-al. The numbering always places "1" on the carbon in the carbonyl group and the rest of the chain is numbered from there. Since the carbon in the carbonyl group is always designated as "1" there is no need to indicate the location of the carbonyl group in the name.
Naming
Ketones
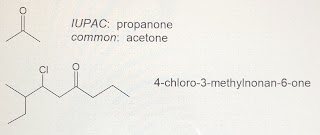
Ketones are named by dropping the ending of the name of the longest chain and adding ‘-one.’ Since the carbonyl group is found within the main chain, we typically need a number to locate its position. When numbering, consider the carbonyl group to be like a substituent and number as usual (so that substituents have the lowest possible numbers).
Ketones are named by dropping the ending of the name of the longest chain and adding ‘-one.’ Since the carbonyl group is found within the main chain, we typically need a number to locate its position. When numbering, consider the carbonyl group to be like a substituent and number as usual (so that substituents have the lowest possible numbers).
Properties
of Aldehydes & Ketones
The properties of aldehydes and ketones are the result of the carbonyl group:
The properties of aldehydes and ketones are the result of the carbonyl group:
O
║
─ C ─
─ C ─
- polar, but cannot H-bond
- higher mp, bp and water-solubility than analogous hydrocarbons
- lower mp, bp and water-solubility than analogous alcohols
Preparing Aldehydes and Ketones from Alcohols: Oxidation Reactions
An oxidation is any chemical process that involves the loss of electrons (we will discuss this further in the Electrochemistry unit). When alcohols are burned in oxygen, complete oxidation occurs and only CO2 and H2O are produced. If the conditions are manipulated, aldehydes and ketones may be formed. Under the special conditions, oxidizing agents are used to provide the O atoms: substances like KMnO4, H2O2 and K2Cr2O7.
In the following equations (O) will be used to represent the active oxygen atom.
In the structures below, I have drawn in hydrogens that we would not normally include in the structure. However, it makes the reaction easier to understand.
- primary alcohol ↦ produces an aldehyde
- secondary alcohol ↦ produces a ketone
- tertiary alcohol ↦ not readily oxidized
 |
| When the active oxygen (O) attacks the tertiary alcohol, there is only the hydrogen in the hydroxyl group. There is no hydrogen on the adjacent carbon, so the reaction cannot proceed. |
From
Aldehydes & Ketones to Alcohols:
Hydrogenation Reactions
Recall that we have seen a hydrogenation reaction in the past. This involved the addition of hydrogen across a C=C double bond. The C=O double bond can also undergo an addition reaction with hydrogen. This is the reverse of the above oxidation reaction.
Recall that we have seen a hydrogenation reaction in the past. This involved the addition of hydrogen across a C=C double bond. The C=O double bond can also undergo an addition reaction with hydrogen. This is the reverse of the above oxidation reaction.
- aldehydes ↦ produce primary alcohols
- ketones ↦ produce secondary alcohols